Eli Lilly reports positive phase 3 results for a weekly insulin formulation
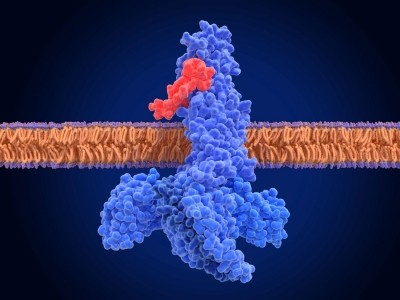
Eli Lilly has announced the results from the QWINT-2 and QWINT-5 clinical trials comparing its once-weekly insulin formulation, efsitora alpha, to the once-daily insulin degludec in adults with type 2 diabetes and type 1 diabetes, respectively. Together, both studies recruited over 1,600 participants across the US, Argentina, Japan, Poland, Puerto Rico, Slovakia and Taiwan, who were treated with either efsitora or degludec over the course of a year.
The primary endpoint of both clinical trials measured the A1C levels in the patients blood, which is used to measure the average levels of blood glucose for the past three months. The data revealed that efsitora achieved a similar reduction of A1C to degludec in both trials.
"People with type 1 diabetes need insulin every day. Currently, they can deliver the insulin using an automated insulin delivery system or by taking a daily basal insulin injection and multiple mealtime insulin injections each day," said Richard Bergenstal, executive director of the International Diabetes Center, HealthPartners Institute. "This new data shows that with one dose a week of basal insulin, efsitora was able to achieve a similar A1C reduction as taking an injection of one of the most used background insulins every day."
Efsitora - the potential to address treatment burden
“Traditionally, basal insulins are dosed once a day — a treatment schedule that can make compliance difficult for a significant portion of people living with type 2 diabetes," added Carol Wysham,clinical professor of medicine at the University of Washington School of Medicine. "Efsitora has the potential to address treatment burden and improve adherence — all while lowering A1C. These results can make a significant impact for people living with type 2 diabetes looking for a once-weekly option that provides similar outcomes as daily insulins.”
However, additional data from the QWINT-5 trial showed that the estimated rate of severe hypoglycemic events, where blood sugar levels fall dangerously low, was higher for efsitora compared to degludec, although the rate of these events at night was similar for both forms of insulin. Lily reported that 64% of these hypoglycemic events took place during the initial 12 weeks of the treatment with efsitora and declined after.
“These results underscore the potential of efsitora to help some people living with type 1 diabetes lower their A1C with only one basal insulin injection per week, while also highlighting the complexity of treating this chronic disease,” said Jeff Emmick, senior vice president of product development at Eli Lilly.
It remains to be seen whether the FDA will greenlight efsitora given the increased rate of hypoglycemic events during the first 12 weeks of treatment. Earlier this year, the FDA rejected an application from Novo Nordisk for another once-weekly insulin formulation, with some of the committee members citing that the benefits of the drug did not outweigh the risks for a higher hypoglycemia rate seen in adults with type 1 diabetes that received the weekly formulation.