FDA approves Galderma’s Nemluvio for chronic skin condition
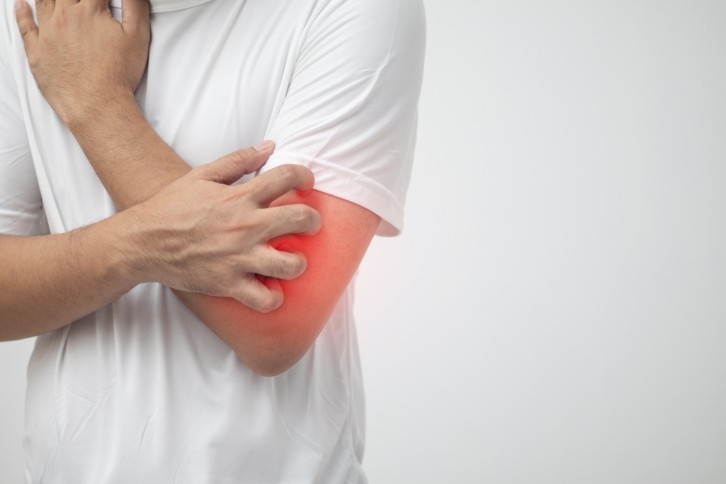
Dermatology specialist Galderma has received FDA approval for Nemluvio (nemolizumab) as a treatment for prurigo nodularis, a chronic skin condition characterized by the development of skin lesions that cause persistent and intense itch. The debilitating symptoms of this neuroimmune disease significantly impact the sleep and quality of life of patients with a prurigo nodularis diagnosis.
Nemluvio is the first approved monoclonal antibody to specifically inhibit the signaling of IL-31, a neuroimmune cytokine that is known for driving multiple disease mechanisms in prurigo nodularis that cause itch, inflammation and fibrosis.
The approval of Nemluvio was based on positive phase 3 results from two OLYMPIA clinical trials, which together enrolled 560 patients with prurigo nodularis. According to Galderma, OLYMPIA is the largest clinical trial program conducted in this condition to date, and the only to include a long-term extension study.
Data from the OLYMPIA trial showed significant and clinically meaningful improvements in itch and skin nodules after 16 weeks of treatment. Nemluvio’s effects in the reduction of itch symptoms were observed as early as the fourth week of treatment.
“The US FDA’s rapid approval of Nemluvio in prurigo nodularis is a first step in achieving its blockbuster platform potential and reinforces our leadership in therapeutic dermatology. We’re confident in the impact this first-in-class therapy will have for patients with prurigo nodularis who urgently need more treatment options and look forward to potentially bringing Nemluvio to patients with other itch-related skin diseases in the near future,” said Flemming Ørnskov, Chief Executive Officer of Galderma.
The FDA is currently reviewing an additional biologics license application (BLA) for Nemluvio for the treatment of moderate-to-severe atopic dermatitis. Galderma expects to receive a response from the FDA on this application later this year.
Marketing authorization applications for Nemluvio in both prurigo nodularis and atopic dermatitis are currently under regulatory review in multiple regions, including the EU, Canada, Australia, Singapore, Switzerland, and the UK. Galderma plans to file additional submissions to other regulatory authorities over the course of the year.
Nemluvio was initially developed by Chugai Pharmaceuticals, a Japanese pharmaceutical company. In 2016, Galderma gained the exclusive rights to the development and marketing of the drug worldwide except for Japan and Taiwan. In Japan, nemolizumab has been approved under the brand name Mitchga since 2022 as a treatment for prurigo nodularis as well as pruritus associated with atopic dermatitis.